The pharmaceutical industry has a history of struggling with data management until the inception of the ALCOA+
framework. Its introduction has transformed the way data is recorded and managed today. In an industry where
there is little tolerance for errors and quality deviations, ALCOA+ has proved to be a boon by allowing the industry
to integrate its data and achieve regulatory compliance.
What is ALCOA+?
ALCOA+ is a system that ensures data integrity in the pharmaceutical manufacturing industry. It is the
brainchild of Food and Drug Administration (FDA) and its main aim is to integrate data and ensure data security.
Adopting ALCOA+ is essential for compliance to FDA guidelines and GMP practices.
Principles of ALCOA
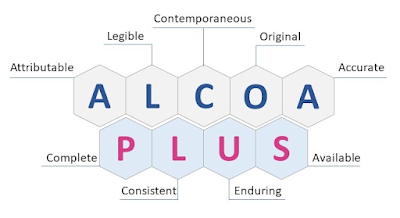
ALCOA is an acronym that stands for
Attributable
Legible
Contemporaneous
Original
Accurate
The ‘+’ at the end of the word indicates the additional elements that are a part of the system, which are complete,
consistent, enduring, and available.
In order to benefit from the system, it’s imperative that you have a thorough understanding of each element, which
will enable you to implement the system successfully and reap its rewards.
So, let’s take a deep dive into the elements that form ALCOA+ and understand each one of them in depth.
Read more, Visit https://www.pharmision.barcodeindia.com/blogs/all-you-need-to-know-about-alcoa/