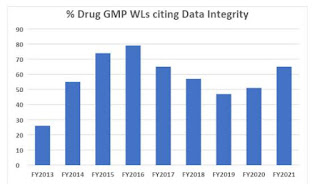
The pharmaceutical industry faces mounting pressure to meet stringent compliance standards by global drug regulators. As a result, the FDA emphasizes the critical role of data integrity in Good Manufacturing Practices. Despite this emphasis, FDA warning letters continue to cite data integrity as a failure, with recent years witnessing an increase in citations after a temporary decrease in 2017-2019.
Although the recent 483s citing data integrity have yet to reach nearly 80%, as witnessed in FY2016, it continues to be a problem that the FDA-governed industry struggles with. Moreover, the deficiencies continue to be the same as they were a decade ago
How can pharma companies ensure data integrity?
To maintain data integrity throughout processes and ensure consistency, organizations can implement fundamental changes that are easy to implement and provide long-lasting benefits. Read more....
Source Link:- https://www.pharmision.barcodeindia.com/blogs/ensuring-data-integrity-in-pharma-companies-practical-steps-to-take-today/